More Information
Submitted: September 29, 2023 | Approved: June 18, 2024 | Published: June 19, 2024
How to cite this article: Bhojraj N, Sreenivasan PK, Gehlot PM, Manjunath V, Manjunath MK. Effectiveness of an Ayurvedic Gel for Tooth Pain Relief Due to Dental Caries: A Randomized Controlled Trial. J Clin Adv Dent. 2024; 8: 013-019.
DOI: 10.29328/journal.jcad.1001041
Copyright License: © 2024 Bhojraj N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Dental pain; Dental caries; Ayurvedic medicine; Visual analog scale
Effectiveness of an Ayurvedic Gel for Tooth Pain Relief Due to Dental Caries: A Randomized Controlled Trial
Nandlal Bhojraj1*, Prem K Sreenivasan1-4, Paras Mull Gehlot5, Vinutha Manjunath5 and Manjunath MK6
1Professor and Head, Department of Pediatric and Preventive Dentistry, JSS Dental College and Hospital, JSS Academy of Higher Education and Research, Mysore, India
2HITLAB, Columbus Avenue, New York, NY, USA
3Rutgers School of Dental Medicine, Newark, NJ, USA
4Adjunct Faculty, JSS Academy of Higher Education and Research, Mysore, India
5Reader, Department of Conservative Dentistry and Endodontics. JSS Dental College and Hospital, JSS Academy of Higher Education and Research, Mysore, India
6Professor and Head (Former), Department of Conservative Dentistry and Endodontics. JSS Dental College and Hospital, JSS Academy of Higher Education and Research, Mysore, India
*Address for Correspondence: Dr. Nandlal Bhojraj, MDS, Professor, Department of Pediatric and Preventive Dentistry, JSS Dental College and Hospital, JSS Academy of Higher Education and Research, (JSSAHER), JSS Medical Institutions Campus, Sri Shivarathreeshwara Nagara, Mysuru 570015, Karnataka, India, Email: [email protected]
Aim: To evaluate the effectiveness of an ayurvedic gel in tooth pain reduction due to dental caries.
Materials and methods: This in vivo cross-over design study enrolled adults with at least one tooth with caries and a symptom of pain after the application of an ice stimulus. Two hundred patients were screened and eligible patients were enrolled in the study. Forty-five subjects completed washout phases before each recall visit. During each recall visit, subjects evaluated pain relief following an ice bar stimulus and one random finger-tip application of a treatment i.e. Ayurveda Herbal gel containing clove oil, camphor, and menthol (Ayurveda Herbal Gel Group), and two control formulations: a gel without active ingredients and commercial olive oil as a surrogate of home remedy. At each assessment, subjects used a stopwatch to record the onset of pain relief and tooth pain using visual analog scores (VAS), dental pain scores (DPS), and relief from tooth pain by dental pain relief scores (DPRS). After each treatment, subjects recorded their satisfaction with the provided treatment using a four-point satisfaction index. Data were tabulated and statistical analysis was performed with (ANCOVA) and two-way ANOVA with a p - value of 0.05 considered statistically significant.
Results: Forty-five subjects (28 males and 17 females) completed the entire study without any adverse events. Application of the Ayurveda gel resulted in a significantly faster onset of pain relief (2.47 min) in comparison to the onset of pain relief after 4 minutes recorded with the controls (p < 0.05). Subjects reported lower VAS and DPS scores over the study period of evaluation when using the Ayurveda gel compared to the application of each control formulation. Subjects also reported greater relief of pain and greater satisfaction after the application of the Ayurveda gel as compared to the controls.
Conclusion: Significantly better tooth pain relief from caries was observed from an Ayurveda Herbal gel than from controls.
Dental caries and periodontal diseases are the most common chronic diseases worldwide [1]. Despite the advances in oral health policies, dental caries remains the most prevalent and costly oral disease, representing a global public health problem to be managed by authorities and dental professionals [2,3].
Dental caries may occur on any tooth surface in the oral cavity where dental plaque is allowed to develop over some time [4]. It is frequently associated with tooth pain and discomfort. Under normal circumstances, any noxious thermal stimulus does not elicit nociception in the teeth due to the insulating capacity of the outer shell of the tooth, the enamel. However, due to dental caries or cervical abrasion, the enamel is lost resulting in the exposure of dentin, and small changes in temperature and light touches such as air puff or water spray can evoke sudden and intense pain in the tooth [5].
Recently there has been increased dialogue related to natural antimicrobials as topical actives and preservatives in the personal care industry [6,7]. The demand for plant-based therapeutics is increasing in both developed and developing countries due to the growing recognition that they are natural products, are nonnarcotic, have no side effects, are easily available at affordable prices, and sometimes the only source of healthcare available to the poor. About 80% of the people in developing countries use traditional medicines for their health care. The natural products derived from medicinal plants have proven to be an abundant source of biologically active compounds, many of which have been the basis for the development of new directions for patient care [8].
Essential oils, including coconut oil, tea tree oil, eucalyptus oil, clove oil, and sesame oil have been widely used in dental practices to treat many oral diseases [9]. Clove oil (Syzygium aromaticum) is applied for toothache, dental caries, and periodontitis [8] and is an active ingredient in several mouthwashes and over-the-counter toothache pain-relief preparations using animal models, the anesthetic effects of eugenol, the main component of clove, as well as its analgesic and anti-inflammatory effects have been well documented [10,11].
However, only a few studies have been validated adequately by either in vitro testing or in vivo clinical trials [11-13]. Hence the aim of this clinical investigation was to evaluate the effects of an ayurvedic formulation on tooth pain due to caries.
The clinical study was a single-center, double-blind, randomized, cross-over design and was conducted at JSS Dental College and Hospital, Mysuru, India after ethical board approval (Ref.No.JSS/DC/ethical/11-12). This prospective study was carried out in 6 months. Study volunteers (age range 18-70 years) of either gender were recruited from the local area. During the initial visit, adult subjects were screened and informed consent was obtained for participation in the study. Subjects were then enrolled in the study based on the inclusion/exclusion criteria.
Inclusion criteria
(i) Males and females in good general health aged 18 to 70 years, who gave informed Consent and had a minimum of 15 natural teeth without crowns or other restorations. (ii) Having adequate oral hygiene and no signs of oral neglect. (iii) Had no clinical and radiographic evidence of periapical involvement. Using the assessment criteria, subjects were enrolled who consistently identified at least one tooth with pain in two recall visits upon exposure of the identified tooth to ice test: A minimum score of 50 millimeters on a 100-millimeter visual analog scale (VAS). Moderate to Severe pain using the four-point Dental Pain Scale (DPS).
Exclusion criteria
(i) History of adverse effects including allergies following use of oral hygiene products such as toothpaste and mouth rinses, gagging or other reflexes that prevented oral examination, (ii) current use of desensitizing formulations, (iii) other treatments for tooth pain or under the care of a health care professional for treatments, (iv) subjects who have used these formulations in the past three months were excluded, (v) subjects using analgesics or anesthetics for 10 days before study enrollment, (vi) teeth that are grossly carious, fully crowned or extensively restored on facial and/or lingual surfaces, (vii) orthodontically banded, abutments, or third molars, (viii) significant oral soft tissue pathology or systemically related gingival enlargement. (ix) History of active severe periodontal disease with bleeding gums and loose teeth (x) gross dental caries, severe generalized cervical abrasion and/or enamel abrasion, large fractured or temporary restorations were not included in the tooth count, (xi) fixed or removable orthodontic appliance or removable partial dentures, (xii) participation in any clinical study within the past three months, (xiii) history of dental prophylaxis or treatments in the past three months, (xiv) self-reported pregnancy or lactation, (xv) history or current use of objects to pierce the lips or tongue, (xvi) subjects known to be an alcoholic, or a recovering alcoholic, history or current use of recreational drugs, history of diabetes or hepatic or renal disease, or other serious medical conditions or transmittable diseases, e.g. heart disease or AIDS. (xvii) Subjects currently under the care of medical professionals including traditional medical practices such as ayurveda, Unani, Siddha, homeopathy, etc., (xviii) history of rheumatic fever, heart murmur, mitral valve prolapse, or other conditions requiring prophylactic antibiotic coverage before invasive dental procedures, subjects on antibiotic, anti-inflammatory or anticoagulant therapy during the month preceding the baseline exam, (xix) presence of concomitant oral pain due to any other condition such as soft-tissue lesions; pain due to surgical procedures or injuries and those that may interfere with this study.
Clinical evaluations
Visual Analog Score (VAS): The VAS is a measurement instrument that evaluates pain over a continuum of values. The subjects marked on the line they feel is representative of their current state of Pain. Dental Pain Scale (DPS):0-None/no discomfort, 1-Mild discomfort, 2-Moderate/marked discomfort, 3-Severe/marked discomfort that lasted more than 10 seconds. Dental Pain Relief Scale (DPRS): 0-no relief,1-a little, 2-some, 3-a lot, 4-complete. Global Satisfaction Assessment: To evaluate the effect of provided treatment on relief from tooth pain using a 5-point scale: 0-fair,1-good, 2-very good,3-excellent,4-at 120 minutes or time of dropout
Procedure
During the screening visit, a dentist evaluated the subject for study enrollment. Evaluations were based on study criteria i.e. initial screening, a health questionnaire, an oral soft tissue evaluation that assessed the soft palate, hard palate, gingival mucosa, buccal mucosa, mucogingival fold areas, tongue, sublingual and submandibular areas, salivary glands, along with the tonsillar and pharyngeal areas. Subjects with tooth pain were screened and qualified subjects who could comply with study schedules were scheduled for further screening visits.
First screening visit
The subject was instructed to identify teeth with pain and instructed on the 100-millimeter Visual Analog Score (VAS) chart.
a. Ice was placed on the teeth and the subject’s VAS scores and Dental Pain Scale (DPS) were evaluated.
b. All subjects with scores that were 50 millimeters or above on the VAS evaluation and moderate to severe pain using the Dental Pain Scale (DPS) were recalled for a second screening visit
Second screening visit:
c. Steps (a) and (b) of the first screening visit were repeated.
d. Subjects with scores that are 50 millimeters or above on the 100-millimeter VAS scale (and are ± 20 mm from their first visit) and moderate to severe pain score by DPS were enrolled in the study.
Enrolled subjects were provided a commercially available fluoride toothpaste and toothbrush for oral hygiene over the study duration. They were instructed to refrain from using any other oral hygiene formulations or treatments for the remainder of the study.
Baseline evaluations
Subjects refrained from oral hygiene, food and drink for 3 hours before their scheduled visit to the dental clinic. Subjects who had VAS scores that were 50 millimeters or above on the VAS evaluation and moderate to severe pain using the Dental Pain Scale (DPS) were evaluated.
Treatments and post-treatment evaluations: Subjects were randomly assigned one of the three test formulations using a computer-generated randomization sequence. Using a weighing balance, 100 milligrams of the formulation were applied to the identified tooth, and the weight of the tube before and after dispensing the formulation was recorded. A stopwatch was started upon treatment application. Subjects completed an immediate post-treatment evaluation of tooth pain using the VAS and DPS scales. Subjects were evaluated at regular intervals by the VAS, dental pain scale (DPS), and dental pain relief scale (DPRS). Evaluations were conducted immediately after treatment and at 15, 30, 60, 90, and 120 minutes post-application. A global satisfaction index was completed at 120 minutes post-application.
This completed the study with the provided treatment. Subjects were instructed to perform twice daily oral hygiene with the provided commercially available fluoride toothpaste and soft-bristled toothbrush until their next scheduled visit 3 days later. After the use of all formulations, a dental examiner conducted a clinical examination of oral hard and soft tissues before concluding their participation in the study.
Forty-five subjects [n = 45, male = 28 and female = 17, age range 18-42 years] who provided informed consent and consistently identified at least one carious tooth with pain after exposure to ice and presented VAS scores of 50 millimeters or above and a moderate to severe pain using the Dental Pain Scale (DPS) were enrolled in the study [14].
Test groups
1. An Ayurveda herbal gel containing clove oil, camphor, and menthol.
2. A placebo gel without clove oil, camphor, and menthol.
3. Commercially available olive oil (FARRELL, Jindal retail (India) Pvt.Ltd.).
The tubes with test formulations were over-wrapped with a white label to ensure that neither the examiner nor the subject was aware of the identity of the product. The preparations were indistinguishable in appearance, taste, and viscosity.
All subjects were followed for adverse events throughout the study. The steps described in baseline and treatment were conducted until the subject completed the entire study with all formulations. Subjects who provided inconsistent VAS or DPS evaluations during the visit were excluded from the study. An oral examination was performed before dismissing them. From the study.
Statistical analysis
A general linear model with subject and treatment effects and baseline as a covariate (ANCOVA) was utilized to evaluate VAS and DPS outcomes. A two-way ANOVA (SPSS – version 22.0 IBM Corp, USA) was utilized for the DPRS, onset of pain relief, and global satisfaction index. Differences between the three study treatments were evaluated by the Tukey-HSD test. Statistical significance for all tests is reported at p < 0.05.
Forty-five (45) subjects completed the entire study without any adverse events.
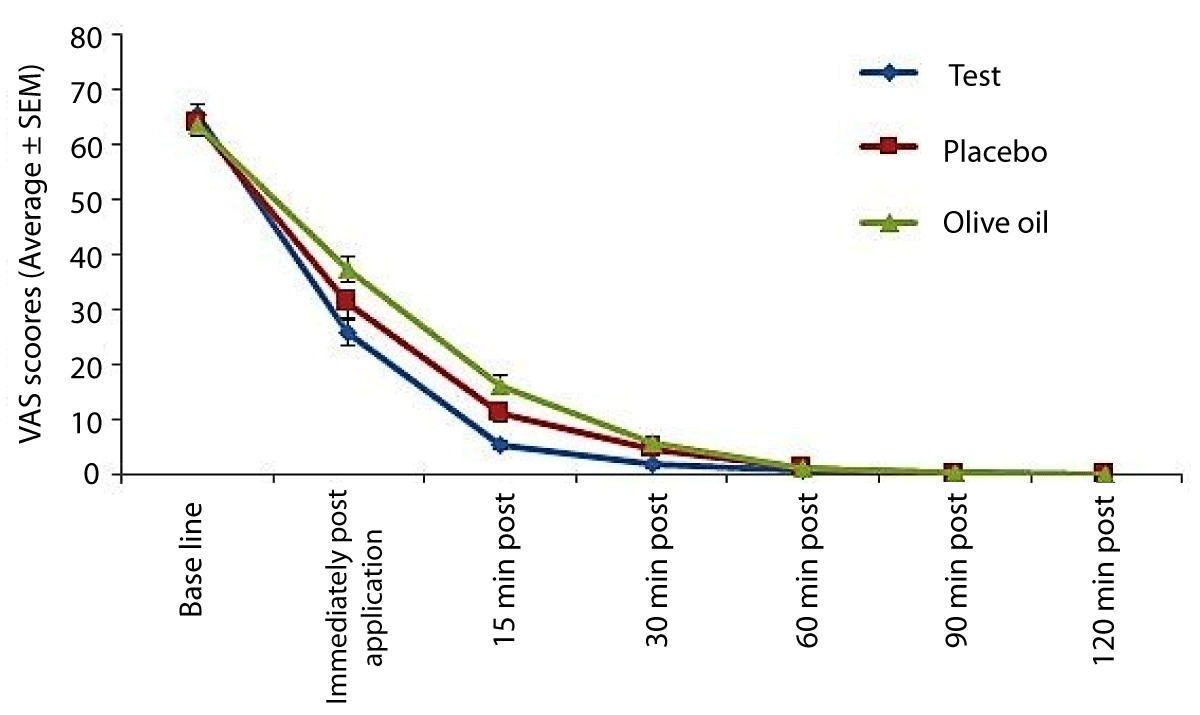
Average VAS scores (Figure 1)
Figure 1: Pain scores amongst subjects recorded on a VAS (Visual Analog Scale) scale. Lower scores indicate pain relief.
Compared to the matching placebo and negative control, the Ayurveda herbal gel resulted in statistically significantly (p < 0.05) lower VAS scores immediately after application, and at the 15-minute and 30-minute post-treatment evaluations. There were no significant differences between treatments at all other evaluations (p > 0.05).
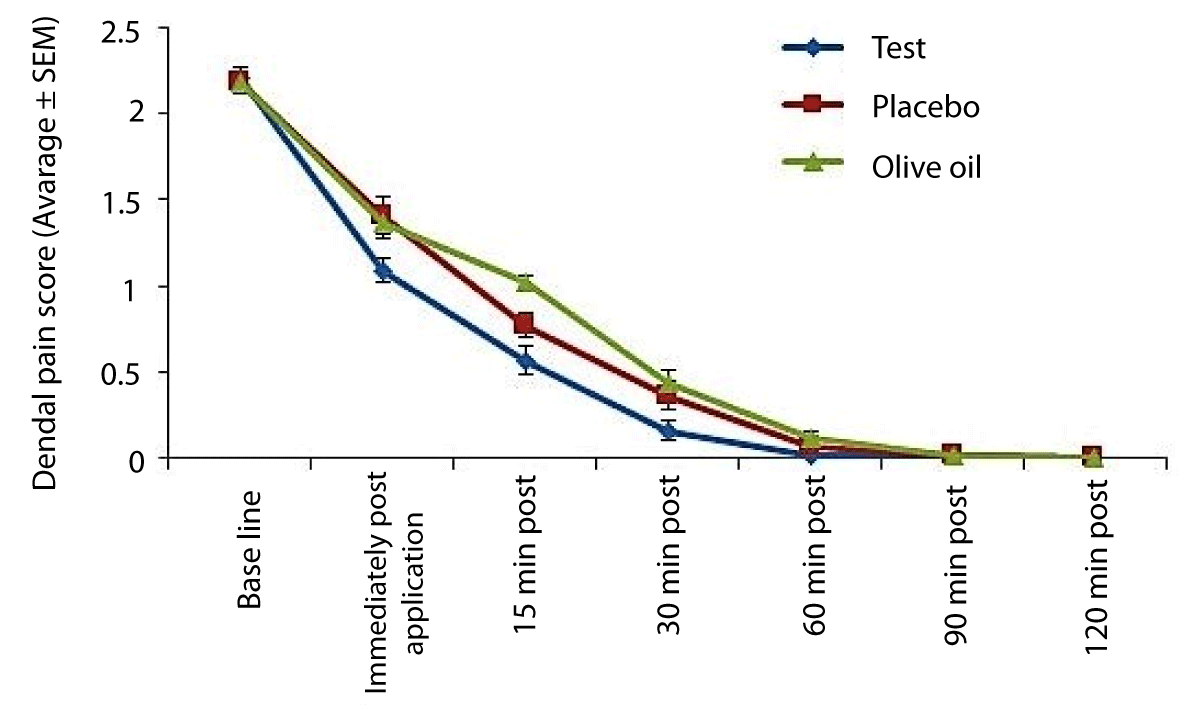
Average DPS scores (Figure 2)
Figure 2: Dental Pain Scores among subjects. The figure indicates average pain scores at each evaluation using a categorical dental pain score (DPS). Lower scores indicate pain relief.
Compared to the matching placebo and negative control, application of the Ayurveda herbal gel resulted in statistically significant (p < 0.05) lower dental pain scores immediately post-application. Differences between the test and controls were not statistically significant (p > 0.05) at all other evaluations.
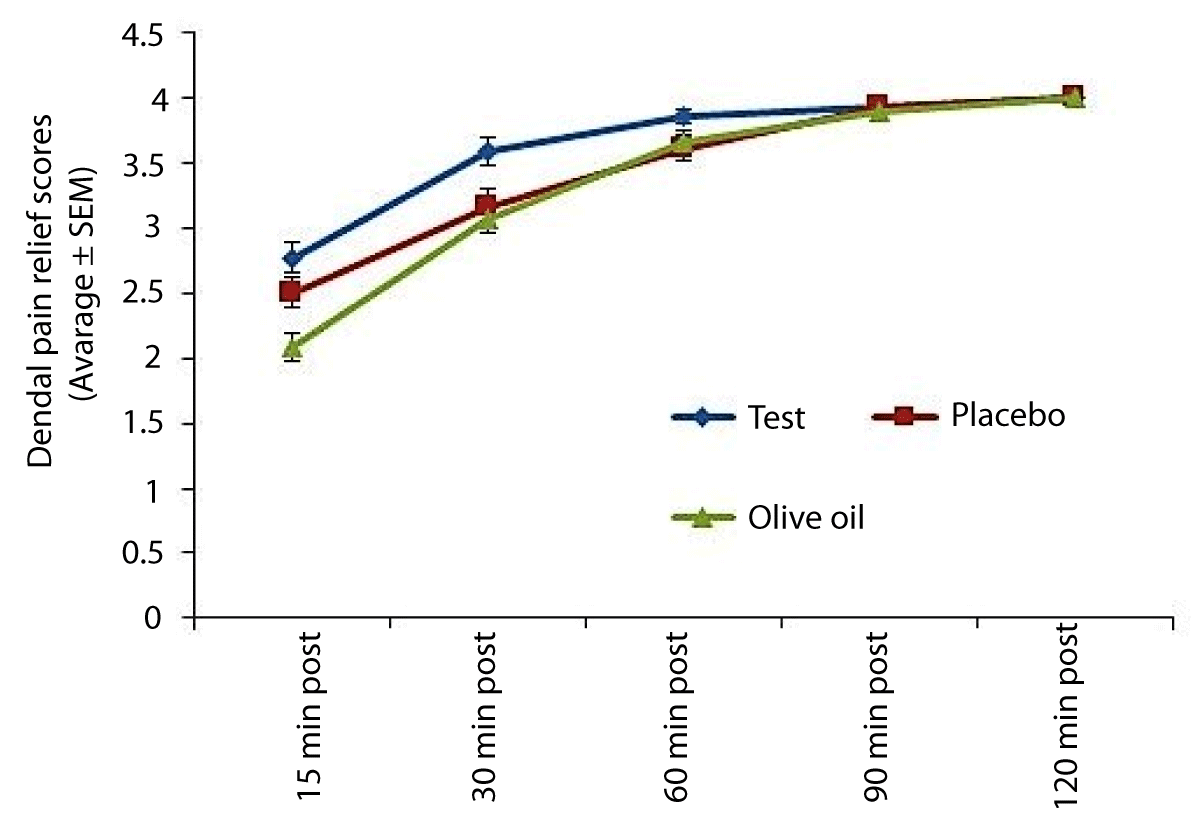
Average DPRS scores (Figure 3)
Figure 3: Pain relief scores amongst subjects. The figure indicates average pain relief at each evaluation using a categorical dental pain relief score (DPRS). Higher scores indicate more pain relief.
The Ayurveda herbal gel product demonstrated significant pain relief in comparison to other treatments at the 30-minute and 60-minute post-treatment evaluations (p < 0.05). There were no significant differences in all other evaluations (p > 0.05).
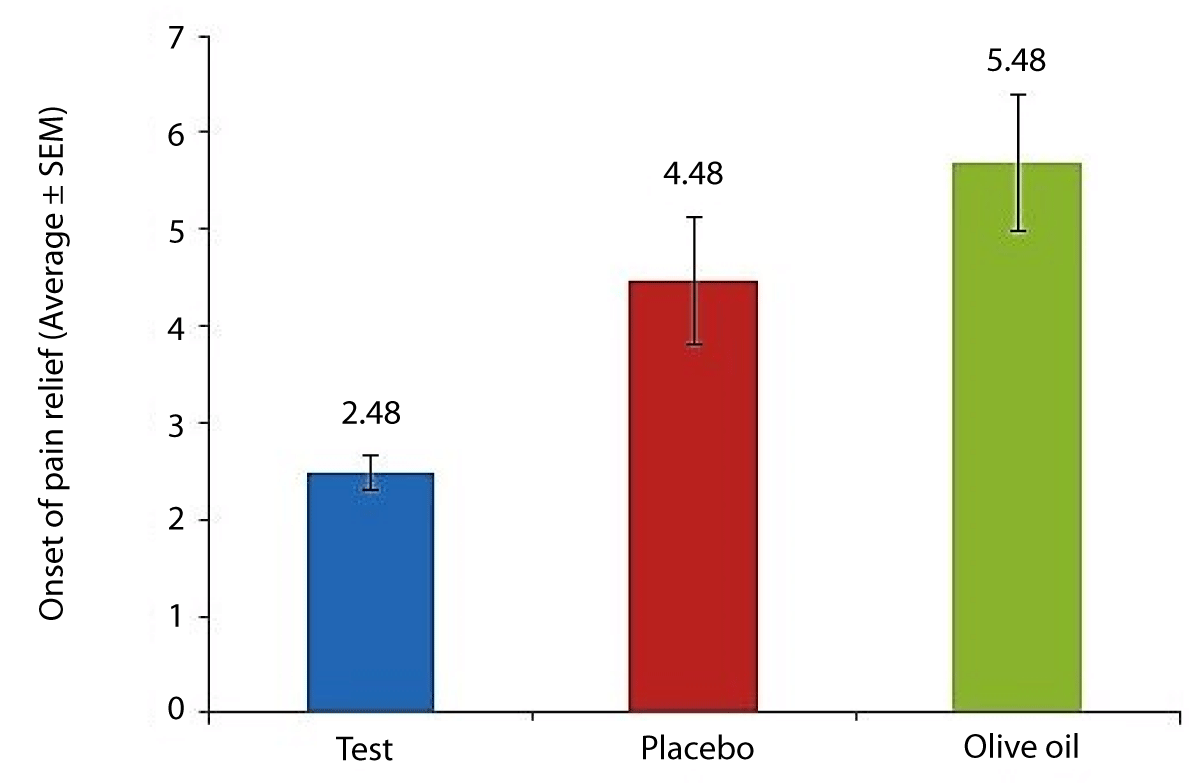
The average time to onset of pain relief (Figure 4)
Figure 4: Average time to onset of pain relief amongst subjects.
For subjects in the Ayurveda herbal gel group [2.48 minutes] was statistically significantly (p < 0.05) lower than subjects in the matching placebo gel and the negative control groups. The time to onset of pain relief by the test was approximately 50% faster for subjects in the Ayurveda herbal gel group compared to subjects provided with the matching placebo gel or the negative control. For each treatment, a frequency distribution for time to onset of pain relief was computed (Table 1). For each treatment, a frequency distribution for the satisfaction index was computed (Table 2).
| Table 1: Frequency distribution for time to onset (in minutes) of pain relief amongst subjects. | ||||
| Treatment | Up to 1 min | Between 1-2 min | Between 2-5 min | More than 5 min |
| Test | 11% | 43% | 43% | 2% |
| Placebo | 0% | 34% | 50% | 16% |
| Olive Oil | 0% | 16% | 59% | 25% |
| Table 2: Satisfaction Index Scores among subjects recorded at the conclusion of each treatment. | ||||
| Treatment | % subjects in each satisfaction score category | |||
| 4 | 3 | 2 | 1 | |
| Test | 7% | 43% | 41% | 9% |
| Placebo | 2% | 27% | 55% | 16% |
| Olive Oil | 0% | 11% | 52% | 36% |
| Satisfaction index score: 0: Fair;1: Good; 2: Very Good; 3: Excellent; 4: At 120 minutes | ||||
Dental caries is reported globally and common in many populations resulting in localized dissolution and destruction of the calcified tissues [1]. It is a chronic disease that involves the destruction of tooth structure, which leads to loss of masticatory function and the unaesthetic appearance of affected teeth. It is manifested by symptoms of pain and sensitivity to hot, cold, and sweet substances [15].
Dental caries is the predominant cause of pulpal inflammation and the pain associated with it. Toxins from the bacteria contained in a carious lesion permeate the dentin and induce inflammation in the pulp long before the bacteria themselves invade the pulp. While carious teeth are commonly painful, in almost a third of cases, the inflammation may progress to necrosis without the patient experiencing discomfort [16].
The pulp has a dense afferent innervation of C fibers and Aδ fibers, which are a dedicated component of the pain system. The Aδ fibers may be those that respond to cold stimuli applied to an uninjured tooth. The C fibers may be silent and only become active in inflammation, causing the dull throbbing pain characteristic of symptomatic pulpitis [17]. The stimuli which initiate dental pain when applied to the dentin, do so mainly by causing fluid movement in the narrow (approx.1–2 μm in diameter) tubules that make up the tissue [18].
Once inflammation is initiated in the pulp, a large number of cytokines and inflammatory mediators are released some of which activate (e.g.bradykinin) or sensitize (prostaglandins) nociceptive terminals. Current evidence supports the central role of neuropeptides in the molecular mechanisms underlying dental pain, in particular, substance P. The major role of substance P in the onset of dental pain and inflammation is increasingly being recognized; and experimental and clinical observations have documented an increase in substance P concentration in patients affected by caries, pulpitis, or granulomas and in those undergoing standard orthodontic or orthodontic/dental care procedures [16,19,20]. Rodd and Boissonade, speculated that the regulation of interdental substance P secretion may provide a new therapeutic approach for the management of dental pain and inflammation [20].
Recently herbal products are staging a comeback and herbal ‘renaissance’ is happening all over the world. These natural products today are popular for safety in contrast to the synthetics that are regarded as unsafe for humans and the environment. Also, having no or minimal side effects, easily available, economical, and sometimes the only source of healthcare available to the poor [21].
For over 2,000 years, both Indian and Chinese traditional medicine made extensive use of clove flowers and clove oil [22]. Cloves have antiseptic, antibacterial, antifungal, and antiviral properties. Cloves (Syzygium aromaticum, Eugenia aromaticum, or Eugenia caryophyllata) the dried flower bud, an evergreen tree 10-20 m. in height Indigenous to India, Indonesia, Zanzibar, Mauritius, and Ceylon They are reddish- brown in colour and have a strong aroma [23]. Although clove oil is sufficiently irritating to preclude general internal usage, it has long been employed as a local analgesic or obtundent for the relief of toothache [24].
Clove contains 14% - 20% of volatile oil that includes eugenol, acetyl eugenol, sesquiterpenes (α-and β-caryophyllenes), and small quantities of esters, ketones, and alcohol. Clove also contains tannins, sitosterol, and stigmosterol [25]. One of the main components of clove oil obtained from the dried flower & buds of S. aromaticum is β-caryophyllene, which has a local anesthetic activity [26].
The essential oil of clove is a colorless or light yellowish extract obtained from dried flower buds by steam distillation. Through Gas chromatography Mass Spectroscopy analysis of clove essential oil 36 components were identified. The highest concentration was of eugenol (88.58%), eugenyl acetate (5.62%), and β-caryophyllene (1.38%) [27] Eugenol is widely used and well-known for its medicinal properties. It is active against oral bacteria associated with dental caries and periodontal disease [28]. The analgesic action elicited by eugenol is the basis for its wide use in dental clinics as a sedative and anodyne agent [29,30]. In a study, ethanol extracts of Syzygium aromaticum flower bud were tested for anti-nociceptive and anti-inflammatory effects in mice and Wistar rats and the result supports the local use of the plant in painful and inflammatory conditions [10].
Studies have confirmed the antinociceptive activity of eugenol against chemical and thermal stimuli. They suggested that eugenol predominantly inhibits or depress the peripheral pain mechanism [11,24,31].
The mechanism of analgesic effect of eugenol could probably be due to the blockade of the effect or the release of endogenous substances that excite pain nerve endings similar to that of indomethacin and other NSAIDs [31]. Eugenol exhibited an efficacy comparable to that of indomethacin in inhibiting neurogenic (first phase) and inflammatory (second phase) pain stimuli caused by formalin test in animal studies [32]. Some studies attribute the analgesic effects of eugenol to its capability to suppress prostaglandins and other inflammatory mediators such as leukotrienes resulting from blockages of the cyclooxygenase and lipoxygenase metabolic pathways [33,34]. The inhibitory action of eugenol on prostaglandin could be attributed to the presence of flavonoids and tannins which were found to inhibit phosphodiesterases [35]. Park, et al examined the antinociceptive profiles of orally administered eugenol in ICR mice and showed an antinociceptive effect in a dose-dependent manner as measured in the acetic acid-induced writhing test. The duration of the antinociceptive action of eugenol was maintained for at least 30 min. The study suggested that eugenol shows an antinociceptive property in various pain models [36]. Additionally, animal studies in mice studies have also revealed eugenol to have the ability to modulate the release of substance P, which has a role in tooth pain as discussed previously [36,37].
A study by Ghelardini, et al. confirmed the in vivo local anesthetic activity of β-caryophyllene in the conjunctival reflex test in rabbits and concluded that the action appears to be strictly dependent on its chemical structure [26]. Lee, et al. concluded that eugenol may have potent anti-inflammatory effects and the application of eugenol during acute pulpal inflammation might alleviate the undesirable consequence of periapical bone resorption and inflammation [38].
Menthol is a naturally occurring compound of plant origin, which gives plants of the Mentha species the typical minty smell and flavour. Menthol is present in the volatile oil of several species of mint plants such as peppermint Mentha piperita and cornmint oil Mentha arvensis [39]. It is widely used in products such as common cold medications, toothpaste, confectionery, and cosmetics.
Transient receptor potential (TRP) channels comprise a group of nonselective calcium-permeable cationic channels, which are polymodal sensors of environmental stimuli such as thermal changes and chemicals. TRPM8 and TRPA1 are cold-sensing TRP channels activated by moderate cooling and noxious cold temperatures, respectively which have been identified in trigeminal ganglion neurons [40]. It has also been reported that modulation of Ca++ currents is involved in the regulation of pain threshold. Inhibition of Ca++ currents by administration of voltage-sensitive Ca++ channel blockers produces antinociception in laboratory animals [41]. Galeotti investigated the potential antinociceptive effect of menthol and concluded, that menthol is endowed with analgesic properties mediated through a selective activation of k opioid receptors. Menthol, after topical application, causes a feeling of coolness due to stimulation of ‘cold’ receptors by inhibiting Ca++ currents of neuronal membranes [39].
Pain is not experienced from an entirely healthy tooth under normal physiological conditions, but can be induced by a cold stimulus at 0°C or below. It is more readily felt in otherwise normal teeth in which the dentin beneath the enamel of the crown or the cementum of the root is exposed [16]. Various methods and materials have been used to test the pulp’s response to thermal stimuli. The baseline or normal response to either hot or cold is a patient’s report that a sensation is felt but disappears immediately upon removal of the thermal stimulus [42]. To mimic the patient’s response to cold stimuli, an ice stick was used [14].
To evaluate patient response to stimuli, a pain scale was applied at the beginning and the end of the experiment so that pain intensity could be quantified and analyzed. The verbal scale used here has been adopted in previous studies [43,44]. Among the three indicators used, verbal descriptors of pain appear to be the best predictors of clinical pain and pain relief. The thermal cold scores, as used in this study, were highly correlated to the verbal descriptors in the prediction of tooth status [14]. In addition, the present study reported a greater global satisfaction index for test products compared to the controls.
The results of the present study cannot be correlated to any previous study, due to the difference in the study parameters. However, limited similar studies have been reported in the literature. A study by Gangarosa, et al. evaluated the effectiveness of benzocaine in relieving pain due to irreversible pulpitis from the carious human tooth [14].
The implication of the above study could be used for formulating an over-the-counter (OTC) product to manage an acute episode of dental pain when the patient is not able to reach/visit the dentist immediately. Eugenol-based products with pain-relieving properties could provide immediate relief to patients without affecting their day-to-day activities until the patient seeks professional advice. However, the limitation of such an OTC product is that it doesn’t provide permanent pain relief to the patient, but is used only as an emergency medicine for acute dental pain.
Results of the present study demonstrate a faster onset of pain relief after the application of the Ayurveda herbal gel than controls. Amongst subjects assigned the test product, significantly lower VAS scores for tooth pain were reported at the 15- and 30-minute post-treatment evaluation than after the application of the control formulations.
- Roberson TM, Heymann HO, Swift EJ. Cariology: the lesion, etiology, prevention and control. In: Sturdevant's Art and Science of Operative Dentistry. 5th ed. New Delhi, India: Mosby Elsevier; 2008; 65-78.
- National Institute of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement. Diagnosis and management of dental caries throughout life, March 26-28, 2001. J Am Dent Assoc. 2001 Aug;132(8):1153-61. doi: 10.14219/jada.archive.2001.0343. PMID: 11575024.
- Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent. 2009 Feb;22(1):3-8. PMID: 19281105.
- Kidd EAM. Essentials of Dental Caries. 3rd ed. New York: Oxford University Press; 2005.
- Chung G, Oh SB. TRP channels in dental pain. Open Pain J. 2013; 6:31-36.
- Shinde SU, Khairnar MR, Wadgave U, Kalghatgi S, Kadam HR. Comparative Efficacy of Analdent (Herbal Analgesic Preparation) and Aceclofenac on Pain Intensity After Tooth Extraction: A Split-Mouth Randomized Clinical Trial. J Maxillofac Oral Surg. 2023 Mar;22(1):152-158. doi: 10.1007/s12663-022-01748-9. Epub 2022 Jun 25. PMID: 36703681; PMCID: PMC9871148.
- Gupta C, Kumari A, Garg AP, Catanzaro R, Marotta F. Comparative study of cinnamon oil and clove oil on some oral microbiota. Acta Biomed. 2011 Dec;82(3):197-9. PMID: 22783715.
- Yadav R, Yadav SK. Dental disease and its cure: a review. Asian J Pharm Clin Res. 2013;6(2):16-20.
- Nuñez L, Aquino MD. Microbicide activity of clove essential oil (Eugenia caryophyllata). Braz J Microbiol. 2012 Oct;43(4):1255-60. doi: 10.1590/S1517-83822012000400003. Epub 2012 Jun 1. PMID: 24031950; PMCID: PMC3769004.
- Tanko Y, Mohammed A, Okasha MA, Umar AH, Magaji RA. Anti-nociceptive and anti-inflammatory activities of ethanol extract of Syzygium aromaticum flower bud in Wistar rats and mice. Afr J Tradit Complement Altern Med. 2008 Jan 22;5(2):209-12. doi: 10.4314/ajtcam.v5i2.31275. PMID: 20161939; PMCID: PMC2816534.
- Daniel AN, Sartoretto SM, Schmidt G, Caparroz-Assef SM, Bersani-Amado CA, Cuman RK. Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Rev Bras Farmacogn. 2009; 19:212-217.
- Kumarswamy A. Multimodal management of dental pain with focus on alternative medicine: A novel herbal dental gel. Contemp Clin Dent. 2016 Apr-Jun;7(2):131-9. doi: 10.4103/0976-237X.183066. PMID: 27307656; PMCID: PMC4906852.
- Chaudhari LK, Jawale BA, Sharma S, Sharma H, Kumar CD, Kulkarni PA. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J Contemp Dent Pract. 2012 Jan 1;13(1):71-4. doi: 10.5005/jp-journals-10024-1098. PMID: 22430697.
- Gangarosa LP Sr, Ciarlone AE, Neaverth EJ, Johnston CA, Snowden JD, Thompson WO. Use of verbal descriptors, thermal scores and electrical pulp testing as predictors of tooth pain before and after application of benzocaine gels into cavities of teeth with pulpitis. Anesth Prog. 1989 Nov-Dec;36(6):272-5. PMID: 2490060; PMCID: PMC2163984.
- Zero DT. Dental caries process. Dent Clin North Am. 1999 Oct;43(4):635-64. PMID: 10553248.
- Holland GR. Dental pain, etiology, pathogenesis and management. In Schmidt RF and Willis WD. Encyclopedia of Pain 2007; 538-540. Springer .New York
- Närhi M, Jyväsjärvi E, Virtanen A, Huopaniemi T, Ngassapa D, Hirvonen T. Role of intradental A- and C-type nerve fibres in dental pain mechanisms. Proc Finn Dent Soc. 1992;88 Suppl 1:507-16. PMID: 1508908.
- Matthews B, Vongsavan N. Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch Oral Biol. 1994;39 Suppl:87S-95S. doi: 10.1016/0003-9969(94)90193-7. PMID: 7702472.
- Sacerdote P, Levrini L. Peripheral mechanisms of dental pain: the role of substance P. Mediators Inflamm. 2012;2012:951920. doi: 10.1155/2012/951920. Epub 2012 Feb 9. PMID: 22474402; PMCID: PMC3306979.
- Rodd HD, Boissonade FM. Substance P expression in human tooth pulp in relation to caries and pain experience. Eur J Oral Sci. 2000 Dec;108(6):467-74. doi: 10.1034/j.1600-0722.2000.00924.x. PMID: 11153921.
- Aneja KR, Joshi R. Antimicrobial Activity of Syzygium aromaticum and Its Bud Oil against Dental Caries Causing Microorganisms. Ethnobotanical Leaflets. 2010; 14: 960-75
- International Organization for Standardization Oil of clove buds [Syzygium aromaticum (Linnaeus) Merril and Perry, syn. Eugenia caryophyllus (Sprengel) Bullock and S. Harrison]. ISO-Directive 3142/1997, Geneva, Switzerland, 2002.
- Blumenthal, M. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin: American Botanical Council, 1998.
- Awang DVC. Tyler's Herbs of Choice: The Therapeutic Use of Phytomedicinals. 3rd ed. CRC Press; 2009.
- Evans WC. Trease and Evans Pharmacognosy. 14th ed. London: Saunders; 2001.
- Ghelardini C, Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A. Local anaesthetic activity of beta-caryophyllene. Farmaco. 2001 May-Jul;56(5-7):387-9. doi: 10.1016/s0014-827x(01)01092-8. PMID: 11482764.
- Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. 2007 Jun;21(6):501-6. doi: 10.1002/ptr.2124. PMID: 17380552.
- Cai L, Wu CD. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod. 1996 Oct;59(10):987-90. doi: 10.1021/np960451q. PMID: 8904847.
- Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, Choi SY, Lee SJ, Lee S, Park K, Lee JH, Kim JS, Oh SB. Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res. 2005 Sep;84(9):848-51. doi: 10.1177/154405910508400913. PMID: 16109996.
- Khan ZA, Prabhu N, Ahmed N, Lal A, Issrani R, Maqsood A, Alam MK, Alanazi S, Aljohani FM, Almndel MN, Alolait MAA. A Comparative Study to Evaluate the Effect of Honey and Zinc Oxide Eugenol Dressing for the Treatment of Dry Socket: A Double-Blind Randomized Controlled Trial. Appl Sci. 2022; 12(6):6.
- Kurian R, Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL. Effect of eugenol on animal models of nociception. Indian J Pharmacol. 2006; 38:341-345.
- Abbott FV, Franklin KBJ, Westbrook FR. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995 Jan;60(1):91-102. doi: 10.1016/0304-3959(94)00095-V. PMID: 7715946.
- Raghavenra H, Diwakr BT, Lokesh BR, Naidu KA. Eugenol--the active principle from cloves inhibits 5-lipoxygenase activity and leukotriene-C4 in human PMNL cells. Prostaglandins Leukot Essent Fatty Acids. 2006 Jan;74(1):23-7. doi: 10.1016/j.plefa.2005.08.006. Epub 2005 Oct 7. PMID: 16216483.
- Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, Park HJ, Nam Han Y, Lee SK. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003 Jun 6;73(3):337-48. doi: 10.1016/s0024-3205(03)00288-1. PMID: 12757841.
- Duke JA. Handbook of medicinal herbs. 2nd Ed. Boca Raton, 2002; FL CRC press
- Park SH, Sim YB, Lee JK, Kim SM, Kang YJ, Jung JS, Suh HW. The analgesic effects and mechanisms of orally administered eugenol. Arch Pharm Res. 2011 Mar;34(3):501-7. doi: 10.1007/s12272-011-0320-z. Epub 2011 May 6. PMID: 21547684.
- Dal Bó W, Luiz AP, Martins DF, Mazzardo-Martins L, Santos AR. Eugenol reduces acute pain in mice by modulating the glutamatergic and tumor necrosis factor alpha (TNF-α) pathways. Fundam Clin Pharmacol. 2013 Oct;27(5):517-25. doi: 10.1111/j.1472-8206.2012.01052.x. Epub 2012 Jul 8. PMID: 22775297.
- Lee YY, Hung SL, Pai SF, Lee YH, Yang SF. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J Endod. 2007 Jun;33(6):698-702. doi: 10.1016/j.joen.2007.02.010. Epub 2007 Apr 2. PMID: 17509409.
- Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002 Apr 12;322(3):145-8. doi: 10.1016/s0304-3940(01)02527-7. PMID: 11897159.
- El Karim IA, Linden GJ, Curtis TM, et al. Human dental pulp fibroblasts express the "cold-sensing" transient receptor potential channels TRPA1 and TRPM8. J Endod. 2011; 37:473-478.
- Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci. 1994 Aug;14(8):4882-90. doi: 10.1523/JNEUROSCI.14-08-04882.1994. PMID: 8046458; PMCID: PMC6577170.
- Berman LH, Hartwell T. Diagnosis in Cohen's Pathways of the Pulp. In: Hargreaves KM, Cohen S, eds. 10th ed. St. Louis, MO: Mosby Elsevier; 2011; 2-39.
- Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: Recent trends in management. J Conserv Dent. 2010 Oct;13(4):218-24. doi: 10.4103/0972-0707.73385. PMID: 21217949; PMCID: PMC3010026.
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997 Nov;24(11):808-13. doi: 10.1111/j.1600-051x.1997.tb01194.x. PMID: 9402502.