More Information
Submitted: September 09, 2024 | Approved: September 19, 2024 | Published: September 20, 2024
How to cite this article: Pulatkan A, Çına M. Comparison of Trigger Point Lidocaine Injection and Stabilization Splint Use in Myofascial Orofacial Pain Treatment. J Clin Adv Dent. 2024; 8(1): 040-046. Available from: https://dx.doi.org/10.29328/journal.jcad.1001045
DOI: 10.29328/journal.jcad.1001045
Copyright License: © 2024 Pulatkan A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Myofascial orofacial pain; Stabilization splint; Trigger point injection
Comparison of Trigger Point Lidocaine Injection and Stabilization Splint Use in Myofascial Orofacial Pain Treatment
Ayşegül Pulatkan1 and Müge Çına2*
and Müge Çına2*
1Başakşehir Oral and Dental Health Center, Istanbul, Turkey
2Oral and Maxillofacial Surgery, Faculty of Dentistry, Süleyman Demirel University, Isparta, Turkey
*Address for Correspondence: Dr. Müge Çına, DDS, PhD, Associate Professor, Oral and Maxillofacial Surgery, Faculty of Dentistry, Süleyman Demirel University, Isparta, Turkey, Email: mugecina@hotmail.com
Objective: This study aims to compare the short-, medium-, and long-term efficacy of trigger point local anesthetic injection and stabilization splint use for myofascial orofacial pain.
Materials and methods: Group 1 comprised 15 patients who received trigger point local anesthetic injections (LAI), while Group 2 comprised 15 patients who were treated with a stabilization splint (SS). Analysis of pain-free maximum mouth opening (MMO) measurements, jaw disability checklists (JDC), short-form McGill pain questionnaires (SF-MPQ), and Visual analog scales (VAS) were used for comparison between the groups.
Results: The LAI group showed a significantly greater increase in pain-free MMO in all terms (p < 0.001) and had significantly lower values on the JDC in both the medium (p = 0.026) and the long term (p = 0.006). The SF-MPQ was significantly lower in the medium term (p = 0.001) in the LAI group; the VAS showed a significantly greater decrease in the short (p = 0.016) and medium terms (p < 0.001) in the LAI group.
Conclusion: The results indicate that a treatment choice can be made between TN lidocaine injection and occlusal splint based on patient tolerance.
Myofascial pain syndrome is characterized by sensory, motor, and autonomic symptoms attributed to trigger points (TrPs). It impedes muscle strength, restricts full muscle elongation, and enhances antagonist muscle contractions [1]. It is one of the most common pain-related temporomandibular disorders (TMDs) in patients with orofacial pain, followed by disc displacement with reduction and arthralgia [2]. Given the high incidence of myofascial pain and the lack of consensus on which treatment modalities are better, a cautious approach from diagnosis to treatment is essential.
It is assumed that TrPs are specific areas in a muscle where sarcomeres contract excessively, resulting in an increase in the diameter of the muscle fibers in that region. It is further assumed that numerous TrPs form palpable contraction knots within tense bands. TrPs that elicit symptoms upon provocation are termed active TrPs, whereas those that do not produce symptoms are termed latent TrPs. Ball, et al. demonstrated that palpable and hyperperfused contraction nodes are formed by non-palpable hypoperfused TrPs in the motor end plate [3]. It is reported that the pathophysiology of TrP formation was initially explained by Simons and Travell in 1981 through the “energy crisis hypothesis,” attributing TrP development to trauma and subsequent myofibril-level damage. This hypothesis has evolved into the widely accepted but controversial “integrated hypothesis,” emphasizing the role of high-level synaptic acetylcholine concentration and intracellular calcium. The impeded dynamic, rhythmic contraction-relaxation cycle prevents capillary circulation, leading to a decrease in nutrient supply and metabolite removal, increasing energy demand and resulting in a decreased pH environment. Increased levels of algogenic substances acting in both orthodromic and antidromic directions at sensory and autonomic terminals lead to sensitization, defined as a decrease in pain threshold and an increase in receptor sensitivity [4,5].
Non-invasive and minimally invasive interventions are employed in the treatment of myofascial orofacial pain (MOP). Occlusal splints are among the non-invasive treatments and include various oral orthotic devices. In the present study, stabilization splints (also known as flat-plane splints, centric relation splints, and Michigan splints) are utilized. Stabilization splints aim to position the mandible in centric relation, eliminate occlusal interferences, and stabilize neuromuscular activity. Their use is thought to potentially decrease temporomandibular joint (TMJ) load and induce changes in proprioception (cognitive awareness theory) [6]. The effectiveness of occlusal splints compared to controls in reducing myogenic TMD symptoms has been demonstrated in many studies [7,8].
Minimally invasive treatments aim to induce regeneration by disrupting the integrity of contracted myofibrils and interrupting pain pathways. In recent years, there has been increased interest in needling therapies, particularly those targeting TrPs through intramuscular injection treatments referred to as TrP injections. The literature reports the use of local anesthetics (LAs), botulinum toxin (BoNT), corticosteroids, platelet-rich plasma (PRP), collagen, sclerosing agents, physiological saline, and granisetron for TrP injections [9]. It assumes that LAs stabilize the membranes not only in axonal transmission but also in muscle fibers, with their effects varying depending on the active substance and concentration [10].
The hypothesis of the present study was that TrP lidocaine injections would be more effective than occlusal stabilization splint therapy in the treatment of myofascial orofacial pain. Accordingly, the aim of this study is to compare the short-term (four weeks), medium-term (three months), and long-term (six months) efficacies of TrP lidocaine injections versus stabilization splints in terms of pain and function.
The research was approved by Süleyman Demirel University Clinical Trials Ethics Committee (date and number, 16.08.2023/165) and performed in accordance with the Declaration of Helsinki Participants who were diagnosed with MOP according to the International Classification of Orofacial Pain (ICOP-1) [11]. ICOP-1 is compatible with the commonly used tool “Research Diagnostic Criteria for Temporomandibular Disorders” (RDC/TMD) but provides a more detailed diagnosis.
All treatments commenced and concluded at the Oral and Maxillofacial Surgery Clinic, Faculty of Dentistry, Süleyman Demirel University, 2022. Inclusion criteria were as follows: minimum age of 18, no previous dry needling or injection therapy applied to the masseter and/or temporalis muscles, no use of occlusal splint in the last six months, no medication affecting muscle relaxation, no systemic neuromuscular diseases, no pregnancy, and obtained informed consent.
The sample size was calculated at a 95% confidence level using the “G. Power-3.1.9.2” program. With a significance level of α=0.05 and a theoretical power of 0.80, the effect size was 0.253. The analysis resulted in a minimum sample size of 24 in total. However, it was decided to include 30 patients in this study. Fifteen patients treated with TrP lidocaine injection formed the local anesthetic injections (LAI) group. The other 15 patients treated with a stabilization splint constituted the stabilization splint (SS) group.
The comparative analysis between the two groups was conducted using a pain-free maximum mouth opening (MAA), Jaw Disability Checklist (JDC), Short Form McGill Pain Questionnaire (SF-MPQ), and Visual Analog Scale (VAS). Follow-up assessments were performed at four weeks (short term), three months (medium term), and six months (long term) post-treatment. Only patients who had completed all measurements and surveys in the examination form at these specific time points were included in this study.
Pain-free MAA was measured using a caliper. When the patients slowly began to open their mouths, they were instructed to stop at the point at which they first felt pain. The distance between the incisal edges of the upper and lower central incisor teeth was then measured using a caliper. The Turkish version of JDC from the RDC/TMD questionnaire was utilized to assess functional reference, and the Turkish version of SF-MPQ and VAS was used to assess pain level and intensity [12-14].
Trp lidocaine injection protocol
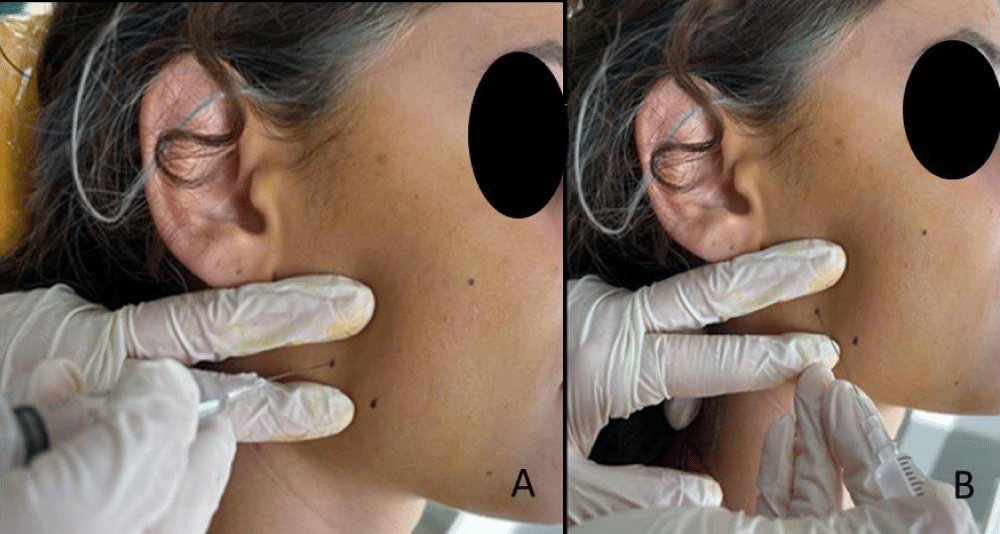
An antiseptic solution (polyvinylpyrrolidone-iodine complex) was applied to the skin in the masseter and/or temporal muscle areas. A 0.5% lidocaine hydrochloride solution (commercial name: Jetocain Simplex 20 mg/ml injection solution) was prepared with physiological serum. Contraction knots within the masseter muscle (excluding the anterior fibers of the muscle) were identified through bilateral palpation. The anterior fibers of the masseter were examined through bidigital palpation. For temporal muscle, only bilateral palpation was performed. A contraction knot containing TrPs located in the taut band was identified. The contraction knot is believed to be the location where pain is experienced. After entering the contraction knot with a 27G disposable hypodermic needle (commercial name: Ayset 2 ml Dental Injector 27G 0.40X50 mm) and ensuring negative aspiration, 0.3 ml of lidocaine hcl solution was injected per each TrPs (Figure 1). The injection needle was partially withdrawn from the skin before completely exiting, and the area around the contraction knot where the solution was administered was mechanically disrupted at several points. Subsequently, the needle was fully removed from the skin, and hemostasis was awaited for 1-2 minutes. This process was repeated for each contraction knot. Injections were repeated once a week at the same points for a duration of four weeks and performed by the same physician.
Figure 1: A,B: Trp lidocaine injection
Stabilization splint protocol
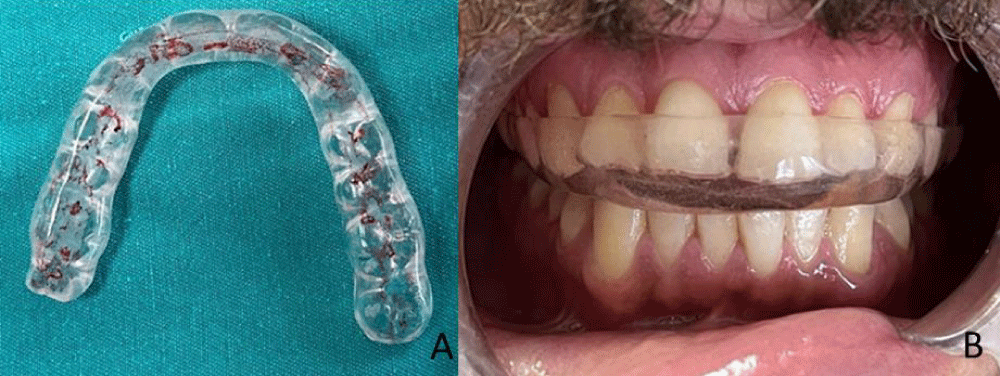
Custom-made hard acrylic material occlusal stabilization splints (2 mm - 4 mm thickness) were used only during sleep for a period of six months (Figure 2). The adjustment of the opposing arch relation during centric and eccentric movements was performed using articulation paper.
Figure 2: A, B: Stabilization splint
Statistical analysis
The data obtained in the study were analyzed using the SPSS 25.0 (Statistical Package for Social Sciences) program. Descriptive statistical methods, including counts, percentages, means, standard deviations, minimum, median, and maximum, were employed for data evaluation. Parametric tests were utilized for measurements with a normal distribution, while non-parametric tests were applied for measurements without a normal distribution. For the comparison of means for two independent groups with a normal distribution of quantitative data, the independent samples t-test was employed, whereas the Mann-Whitney U analysis was used for non-normally distributed measurements. In the case of finding significant differences, Bonferroni analysis was conducted to identify specific pairwise differences. The Type I Error probability (α) was set at 0.05 for all analyses.
The present study included 30 (27 women, 3 men) patients aged 30.93 ± 10.85 years (range 18-65). Based on diagnoses, 13.3% were acute primary MOP (n = 4), 76.7% were chronic frequent primary MOP with pain referral (n = 23), and 10% were chronic highly frequent primary MOP with pain referral (n = 3).
Pain-free maximum mouth opening (MMO) measurements
Both groups showed statistically significant differences at four weeks, three months, and six months compared to pre-treatment values. No significant difference was observed in pre-treatment between the treatment groups. Statistically significant differences between the two groups were observed at four weeks (p < 0.001), three months (p < 0.001), and six months (p < 0.001) after treatment. The LAI group had higher mean MMO values than the SS group (Table 1).
| Table 1: Pre- and post-treatment scores of pain-free MMO. | |||||
| LAI group (n = 15) | SS group (n = 15) | ||||
| Median (Min-Max) |
Mean ± SD | Median (Min-Max) |
Mean ± SD | p | |
| Pre-treatment (0) | 25(8-36) | 25.67 ± 7.31 | 27(14-34) | 27.53 ± 5.29 | 0.430 |
| 4 weeks (1) | 45(35-54) | 44.60 ± 5.07 | 36(25-43) | 35.60 ± 4.36 | 0.000* |
| 3 months (2) | 46(34-52) | 45.53 ± 3.96 | 35(25-47) | 36.33 ± 6.61 | 0.000* |
| 6 months (3) | 46(42-53) | 46.33 ± 2.69 | 35(25-47) | 36.20 ± 7.28 | 0.000* |
| Bonferroni (p) | 0 < 1 (0.001) 0 < 2 (0.000) 0 < 3 (0.000) |
0 < 1 (0.001) 0 < 2 (0.001) 0 < 3 (0.002) |
|||
| Mann Whitney U test, Bonferroni test * p < 0.001 | |||||
Jaw Disability Checklist (JDC)
Before treatment and after four weeks (p = 0.014), three months (p < 0.001), and six months (p < 0.001), statistically significant differences were observed in LAI group JDC scores. In the SS group, a significant difference was only shown at three months (p = 0.022).
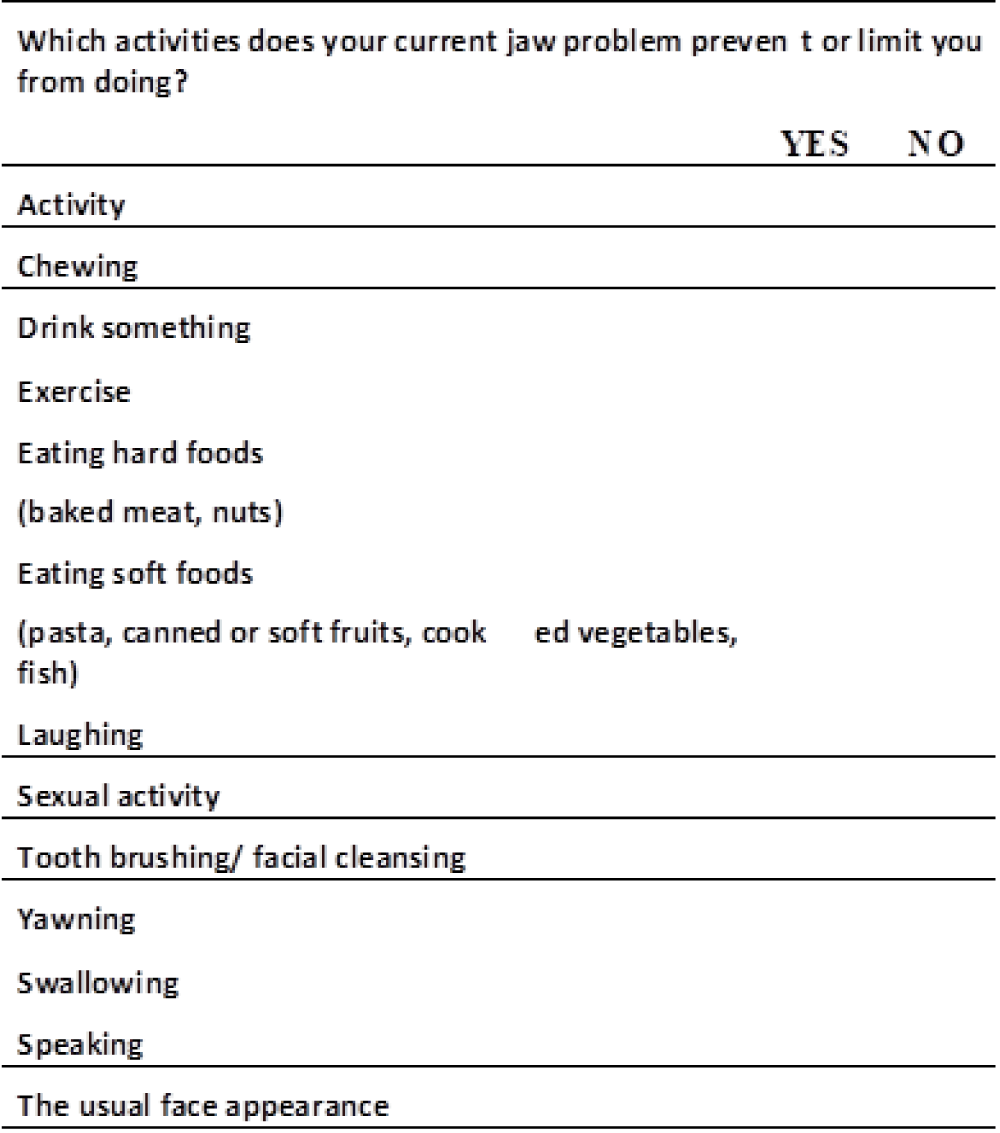
There was no statistically significant difference in the mean JDC scores between the groups before (p = 0.099) and after four weeks of treatment (p = 0.093). For both groups, mean JDC scores showed a significant difference at three months (p = 0.026) and six months (p = 0.006) after treatment; the LAI group had lower values (Figure 3) (Table 2).
Figure 3: Jaw Disability Checklist. 1 point for every YES answer.
| Table 2: Pre- and post-treatment scores of JDC. | |||||
| LAI group (n = 15) | SS group (n = 15) | ||||
| Median (Min-Max) |
Mean ± SD | Median (Min-Max) |
Mean ± SD | p | |
| Pre- teratment (0) | 5(2-8) | 5.00 ± 1.69 | 4(2-9) | 4.13 ± 1.68 | 0.099 |
| 4 weeks (1) | 1(0-7) | 1.80 ± 2.08 | 2(0-7) | 2.86 ± 2.29 | 0.093 |
| 3 months (2) | 0(0-4) | 0.93 ± 1.22 | 2(0-6) | 2.40 ± 2.03 | 0.026* |
| 6. months (3) | 0(0-4) | 0.87 ± 1.25 | 3(0-7) | 2.93 ± 2.12 | 0.006* |
| Bonferroni (p) | 1 < 0 (0.014) 2 < 0 (0.000) 3 < 0 (0.000) |
2 < 0 (0.022) | |||
| Mann Whitney U test, Bonferroni test * p < 0.001 | |||||
Short Form McGill Pain Questionnaire (SF-MPQ)
In both groups, a statistically significant difference was observed in the mean SF-MPQ scores before and after all time periods, with the mean values being lower than pre-treatment. Differences between the groups were only observed three months (p = 0.001) after treatment; the LAI group had lower values than the SS group (Figure 4) (Table 3).
Figure 4: Short Form McGill Pain Questionnaire.
| Table 3: Pre- and post-treatment scores of SF-MPQ. | |||||
| LAI group (n = 15) | SS group (n = 15) | ||||
| Median (Min-Max) |
Mean ± SD | Median (Min-Max) |
Mean ± SD | p | |
| Pre-treatment (0) | 18(7-38) | 19.33 ± 10.74 | 14(6-23) | 15.07 ± 5.12 | 0.180 |
| 4 weeks (1) | 2(0-24) | 4.47 ± 7.37 | 3(0-20) | 5.93 ± 5.65 | 0.097 |
| 3 months (2) | 0(0-6) | 1.53 ± 2.23 | 4(1-11) | 5.33 ± 3.42 | 0.001* |
| 6 months (3) | 0(0-11) | 2.13 ± 3.62 | 4(0-16) | 5.13 ± 5.49 | 0.105 |
| Bonferroni (p) | 1 < 0 (0.003) 2 < 0 (0.000) 3 < 0 (0.000) |
1 < 0 (0.001) 2 < 0 (0.011) 3 < 0 (0.000) |
|||
| Mann Whitney U test, Bonferroni test * p < 0.001 | |||||
Visual Analog Scale (VAS)
Before and after all time periods, the mean VAS values were significantly different in both groups. There was a significant difference between the groups at four weeks (p = 0.016) and three months (p < 0.001) on behalf of the LAI group (Figure 5) (Table 4).
Figure 5: Visual Analog Scale
| Table 4: Pre- and post-treatment scores of VAS. | |||||||
| LAI group (n = 15) | SS group (n = 15) | ||||||
| Median (Min-Max) |
Mean ± SD | Median (Min-Max) |
Mean ± SD | p | |||
| Pre-treatment (0) | 7(4-10) | 7.00 ± 1.85 | 7(4-10) | 6.93 ± 2.02 | 0.926 | ||
| 4 weeks (1) | 0(0-6) | 1.60 ± 2.20 | 3(0-6) | 3.40 ± 1.88 | 0.016* | ||
| 3 months (2) | 0(0-3) | 0.47 ± 0.92 | 2(0-5) | 2.33 ± 1.63 | 0.000* | ||
| 6. months (3) | 0(0-7) | 1.33 ± 2.29 | 3(0-6) | 2.60 ± 2.16 | 0.100 | ||
| Bonferroni (p) | 1 < 0 (0.001) 2 < 0 (0.000) 3 < 0 (0.000) |
1 < 0 (0.043) 2 < 0 (0.001) 3 < 0 (0.000) |
|||||
| Mann Whitney U test, Bonferroni test * p < 0.001 | |||||||
Myofascial pain affects 30% to 85% of the population [15]. From 85% to 93% of myofascial pain patients have been reported in pain clinics, with a higher incidence observed in women. In patients with myofascial TMD, the masseter and temporalis muscles are the most common sites for active TrPs [16].
The fact that myofascial pain has common risk factors with some clinical conditions and disorders (emotional state, fatigue, sleep disorders, and parafunctional activities) and the evaluation of comorbidity conditions is important for treatment success. Although the relationship between bruxism and TMD has not been clarified, bruxism is thought to contribute to myofascial pain. There are not many studies in the literature evaluating the presence of bruxism in patients with TrPs. Our study is important in this respect. Polysomnography is the gold standard for the diagnosis of bruxism. The most important limitation of our study is that the diagnosis of bruxism was made clinically due to the fact that the study was conducted retrospectively. However, due to practical difficulties, studies, including bruxism are mostly based on patient declaration [17].
A meta-analysis conducted by Al-Moraissi, et al. reported that the most effective treatment options, in order of effectiveness, are manual therapy, counseling therapy, local anesthetic injection, and occlusal appliance [18]. However, it has been observed in studies comparing minimally invasive and occlusal splint treatments in the literature that the follow-up length is relatively short [19,20]. Blasco-Bonora, et al. observed a significant improvement in pain-free MAA, JDC, and VAS values compared to pre-treatment after dry needling therapy applied to active TrPs in the masseter and temporal muscles in patients with bruxism and myofascial pain at the end of a week. The results of our study also show that significant positive changes in pain-free MAA, JDC, SF-MPQ, and VAS were obtained in all terms in the lidocaine injection group [21].
In this study, both groups demonstrated improvement in all variables (pain-free MMO, VAS, SF-MPQ) except for the JDC at all time intervals. In the long term, there were no significant differences between the groups in terms of VAS and SF-MPQ; however, pain-free MMO and JDC showed a significant difference between the groups in favor of the lidocaine group. The observed difference in the comparative analyses underscores the need for studies comparing minimally invasive and occlusal splint therapies in terms of long-term efficacy.
The obtained results seen in the lidocaine group are thought to be due to a series of events that can be explained by the disruption of TrP integrity, a decrease in Ach levels, and the prevention of a set of events; interruption of sensitization pathways through stabilization of afferent and efferent membranes; and improvement through effects such as direct vasodilation in the region. Ahmed, et al. reported that the effect size of LA injection into TME muscles was high [15]. Xie, et al. stated that injecting lidocaine into TrP and Trp-related motor end plates significantly reduced pain compared to a direct lidocaine injection into the TrP. Despite the lack of imaging-guided injections in our study, we suggest that the observed improvement in the lidocaine group from the fourth week onwards may be attributed to the involvement of motor end plates along with TrP [22].
Several minimally invasive techniques have been described in the literature. Due to its low cost and lower complication ratios, lidocaine is one of the most preferred substances for pain management. Hosgor, et al. reported that following BoNT injections in patients diagnosed with myofascial pain and clinical bruxism, pain-free MMO, and VAS values at one, three, and six-month follow-ups demonstrated a positive difference compared to pre-treatment, similar to another study’s lidocaine group [23]. Given the reversible nature of the paralysis induced by BoNT, the reflected antinociceptive effect on VAS values is an outcome that we would expect. Similar results obtained in our lidocaine group in this study may potentially overshadow the impact of contractility. Canales, et al. also reported no significant difference in symptoms among different doses of BoNT, suggesting the presence of an unproven afferent effect alongside its efferent effect [24].
SF-MPQ and VAS mean values increased in the lidocaine group between the medium and long term. Although these values were significantly lower than the pre-treatment period, the increased mean values between these time intervals explain the absence of significant differences between the groups in the long term.
Vrbanović, et al. observed a significant reduction in symptoms after six months of using a stabilization splint in TMD patients. No significant difference was observed between groups with myofascial pain and those with disc displacement, nor between groups with acute pains and those with chronic pains [25]. We are of the opinion that the use of a stabilization splint is beneficial in cases associated with bruxism. However, to reach a definitive conclusion in this regard, studies utilizing subgroup classifications recommended by ICOP and definitively diagnosing bruxism with PSG are needed.
In a meta-analysis evaluating the effectiveness of occlusal splints in patients with myogenous TMD, it was reported that the most effective occlusal splint for pain is the anterior splint, followed by the combination of counseling therapy and hard stabilization splint. The most effective treatments for achieving mouth opening were noted to be midline stop appliances, counseling therapy, and a hard stabilization splint. Subgroup analyses demonstrated that a hard stabilization splint provided pain relief in the short-term follow-up and when worn only during the night [7]. In our study, the observed significant changes in pain-free MAA, VAS, and SF-MPQ results in the stabilization splint group are consistent with the literature.
Ozkan, et al. concluded that TrP injection therapy, in combination with splint therapy, is more effective than splint therapy alone for the management of TMD. The combination therapy group (Trps lidocaine injection+stabilization splint) showed better VAS results but no significant difference in the maximum incisal opening [26]. Bilici, et al. observed that combination therapy decreased VAS compared to occlusal splints. Increasing number of injections has positive effects on treatment [27]. The absence of a combination group in our study is a limitation of the study.
In the study by Kamanlı, et al. improvement in depression and anxiety measurements was observed following BoNT injections, whereas no effect was observed in the comparison groups receiving LA or placebo injections. This study draws attention to the potential variability in the clinical profile and treatment response of patients with myofascial pain [28]. The success of treatment in patients with muscle-related TMD has been reported to exhibit stronger correlations with changes in jaw movements, fatigue, weakness, individual patient characteristics (psychosocial and behavioral factors), and comorbidities (such as depression, anxiety, and somatization) when compared to changes in pain intensity. Therefore, pain management is likely to be more effective when treatment is personalized after evaluating both the physical and non-physical components of the pain in question [29,30].
The results indicate that a treatment choice can be made between TN lidocaine injection and occlusal splint based on patient tolerance. Variables related to comorbidities such as bruxism should be included in future studies. It is necessary to conduct studies with a larger sample size to provide high-quality evidence.
- Nowak Z, Chęciński M, Nitecka-Buchta A, Bulanda S, Ilczuk-Rypuła D, Postek-Stefańska L, et al. Intramuscular Injections and Dry Needling within Masticatory Muscles in Management of Myofascial Pain: Systematic Review of Clinical Trials. Int J Environ Res Public Health. 2021;18(18):9552. Available from: https://doi.org/10.3390/ijerph18189552
- Serrano-Hernanz G, Angulo-Carrere T, Ardizone-García I, Svensson P, Álvarez-Méndez AM. Pressure release technique versus placebo applied to cervical and masticatory muscles in patients with chronic painful myofascial temporomandibular disorder: A randomized clinical trial. J Oral Rehabil. 2023;50(9):782-791. Available from: https://doi.org/10.1111/joor.13490
- Ball A, Perreault T, Fernández-de-las-Peñas C, Agnone M, Spennato J. Ultrasound Confirmation of the Multiple Loci Hypothesis of the Myofascial Trigger Point and the Diagnostic Importance of Specificity in the Elicitation of the Local Twitch Response. Diagnostics. 2022;12(2):321. Available from: https://doi.org/10.3390/diagnostics12020321
- Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: An application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12(4):371-384. Available from: https://doi.org/10.1016/j.jbmt.2008.06.006
- Donnelly JM. Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual, 3rd ed. 2019 Jan 1. Available from: https://meded.lwwhealthlibrary.com/book.aspx?bookid=3060.
- Bhargava D, Chávez Farías C, Ardizone García I, Mercuri LG, Bergman S, Pogrel MA, et al. Recommendations on the Use of Oral Orthotic Occlusal Appliance Therapy for Temporomandibular Joint Disorders: Current Evidence and Clinical Practice. J Maxillofac Oral Surg. 2023;22(3):579-589. Available from: https://doi.org/10.1007/s12663-023-01939-y
- Al-Moraissi EA, Farea R, Qasem KA, Al-Wadeai MS, Al-Sabahi ME, Al-Iryani GM. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2020;49(8):1042-1056. Available from: https://doi.org/10.1016/j.ijom.2020.01.004
- Alkhutari AS, Alyahya A, Rodrigues Conti PC, Christidis N, Al-Moraissi EA. Is the therapeutic effect of occlusal stabilization appliances more than just placebo effect in the management of painful temporomandibular disorders? A network meta-analysis of randomized clinical trials. J Prosthet Dent. 2021;126(1):24-32. Available from: https://doi.org/10.1016/j.prosdent.2020.08.015
- Machado E, Machado P, Wandscher VF, Marchionatti AME, Zanatta FB, Kaizer OB. A systematic review of different substance injection and dry needling for treatment of temporomandibular myofascial pain. Int J Oral Maxillofac Surg. 2018;47(11):1420-1432. Available from: https://doi.org/10.1016/j.ijom.2018.05.003
- Karadaş Ö, Gül HL, Inan LE. Lidocaine injection of pericranial myofascial trigger points in the treatment of frequent episodic tension-type headache. J Headache Pain. 2013;14(1):44. https://doi.org/10.1186/1129-2377-14-44
- International Classification of Orofacial Pain, 1st edition (ICOP), 2020. Available from: Available from: https://journals.sagepub.com/doi/10.1177/0333102419893823.
- Demir MG. Investigation of maximum mouth opening in terms of age, weight, height and body mass index in Turkish adult population. Cranio. 2023;21:1-6. https://doi.org/10.1080/08869634.2023.2226828
- Turkish | International RDC-TMD Consortium. Available from: https://ubwp.buffalo.edu/rdc-tmdinternational/tmd-assessmentdiagnosis/rdc-tmd/translations/turkish/.
- Biçici B. Investigation of the validity and reliability of ‘Mcgill Pain Scale Short Form’ [Master's thesis]. Ege University Institute of Health Sciences; 2010. Available from: https://tez.yok.gov.tr/UlusalTezMerkezi/TezGoster?key=EEdeQgIdFRxX5NbvVau-ArYRzdJ_pYUs-kuhMSAwHPUSZo_Q454J8r4OlE7QaDkw.
- Ahmed S, Subramaniam S, Sidhu K, Khattab S, Singh D, Babineau J, et al. Effect of Local Anesthetic Versus Botulinum Toxin-A Injections for Myofascial Pain Disorders: A Systematic Review and Meta-Analysis. Clin J Pain. 2019;35(4):353-367. Available from: https://doi.org/10.1097/ajp.0000000000000681
- Galasso A, Urits I, An D, Nguyen D, Borchart M, Yazdi C, et al. A Comprehensive Review of the Treatment and Management of Myofascial Pain Syndrome. Curr Pain Headache Rep. 2020;24(8):43. Available from: https://doi.org/10.1007/s11916-020-00877-5
- Stuginski-Barbosa J, Porporatti AL, Costa YM, Svensson P, Conti PCR. Agreement of the International Classification of Sleep Disorders Criteria with polysomnography for sleep bruxism diagnosis: A preliminary study. J Prosthet Dent. 2017;117(1):61-66. Available from: https://doi.org/10.1016/j.prosdent.2016.01.035
- Al-Moraissi EA, Conti PCR, Alyahya A, Alkebsi K, Elsharkawy A, Christidis N. The hierarchy of different treatments for myogenous temporomandibular disorders: a systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac Surg. 2022;26(4):519-533. Available from: https://doi.org/10.1007/s10006-021-01009-y
- Cássia Maria Grillo, Giancarlo De la Torre Canales, Ronaldo Seichi Wada, Marcelo Corrêa Alves, Célia Marisa Rizzatti Barbosa, Fausto Berzin, et al. Could acupuncture be useful in the treatment of temporomandibular dysfunction? J Acupunct Meridian Stud. 2015 Aug;8(4):192-199. Available from: https://www.sciencedirect.com/science/article/pii/S2005290114002301?via%3Dihub.
- Vicente-Barrero M, Yu-Lu SL, Zhang B, Bocanegra-Pérez S, Durán-Moreno D, López-Márquez A, et al. The efficacy of acupuncture and decompression splints in the treatment of temporomandibular joint pain-dysfunction syndrome. Med Oral Patol Oral Cir Bucal. 2012;17(6):1028-1033. Available from: https://doi.org/10.4317/medoral.17567
- Blasco-Bonora PM, Martín-Pintado-Zugasti A. Effects of Myofascial Trigger Point Dry Needling in Patients with Sleep Bruxism and Temporomandibular Disorders: A Prospective Case Series. Acupunct Med. 2017;35(1):69-74. Available from: https://doi.org/10.1136/acupmed-2016-011102
- Xie P, Qin B, Yang F, Yu T, Yu J, Wang J, et al. Lidocaine Injection in the Intramuscular Innervation Zone Can Effectively Treat Chronic Neck Pain Caused by MTrPs in the Trapezius Muscle. Pain Physician. 2015;18(5):815-826. Available from: https://pubmed.ncbi.nlm.nih.gov/26431135/
- Hosgor H, Altindis S. Efficacy of botulinum toxin in the management of temporomandibular myofascial pain and sleep bruxism. J Korean Assoc Oral Maxillofac Surg. 2020;46(5):335-340. Available from: https://doi.org/10.5125/jkaoms.2020.46.5.335
- De la Torre Canales G, Alvarez-Pinzon N, Muñoz-Lora VRM, Vieira Peroni L, Farias Gomes A, Sánchez-Ayala A, et al. Efficacy and Safety of Botulinum Toxin Type A on Persistent Myofascial Pain: A Randomized Clinical Trial. Toxins. 2020;12(6):395. Available from: https://doi.org/10.3390/toxins12060395
- Vrbanović E, Alajbeg IZ. Long-term Effectiveness of Occlusal Splint Therapy Compared to Placebo in Patients with Chronic Temporomandibular Disorders. Acta Stomatol Croat. 2019;53(3):195-206. Available from: https://doi.org/10.15644/asc53/3/1
- Ozkan F, Cakir Ozkan N, Erkorkmaz U. Trigger point injection therapy in the management of myofascial temporomandibular pain. J Turk Soc Algol. 2011;23(3):119-125. Available from: https://doi.org/10.5505/agri.2011.04796
- Bilici IŞ, Emes Y, Aybar B, Yalçın S. Evaluation of the effects of occlusal splint, trigger point injection and arthrocentesis in the treatment of internal derangement patients with myofascial pain disorders. J Cranio-Maxillofac Surg. 2018;46(6):916-922. Available from: https://doi.org/10.1016/j.jcms.2018.03.018
- Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayık Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25(8):604-611. Available from: https://doi.org/10.1007/s00296-004-0485-6
- Thottungal A, Kumar P, Bhaskar A. Interventions for myofascial pain syndrome in cancer pain: recent advances: why, when, where and how. Curr Opin Support Palliat Care. 2019;13(3):262-269. Available from: https://doi.org/10.1097/spc.0000000000000446
- Wiechens B, Paschereit S, Hampe T, Wassmann T, Gersdorff N, Bürgers R. Changes in Maximum Mandibular Mobility Due to Splint Therapy in Patients with Temporomandibular Disorders. Healthcare. 2022;10(6):1070. Available from: https://doi.org/10.3390/healthcare10061070